CHEMICAL ENERGETICS / THERMOCHEMISTRY / THERMOCHEMICAL EQUATION
CHEMICAL ENERGETICS: IT IS DEFINE AS THE STUDY OF HEAT CHANGE THAT TOOK PLACE WHEN REACTANTS REACTS TO FORM PRODUCT IN A CHEMICAL REACTION AND IT ALSO DEALS WITH EFFECT OF CHANGE IN TEMPERATURE, PRESSURE, CONCENTRATION ON THE ENERGY CHANGE IN CHEMICAL REACTION. CHEMICAL ENERGETICS DISCUSSESS BOTH THERMALDYNAMICS AND KINETICS. IT PROVIDES AN IMPORTANT INFORMATION REGARDING BOND ENERGIES.
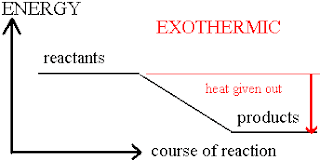
EXOTHERMIC REACTION: IT IS A TYPE OF CHEMICAL REACTION IN WHICH LARGE AMOUNT OF ENERGY IS RELEASED.
ENDOTHERMIC REACTION: IT IS A LYPE OF CHEMICAL REACTION IN WHICH LARGE AMOUNT OF ENERGY IS ABSORBED.
THERMOCHEMISTRY: AS CHEMICAL ENERGETICS DEALS WITH THE STUDY OF HEATS CHANGE SIMILARLY THERMOCHEMISTRY DEALS WITH THE STUDY OF ENERGY TRANSFORMATION AS HEAT IS ALSO A FORM OF ENERGY. A REACTION MAY RELEASE OR ABSORB ENERGY , AND A PHASE CHANGE MAY DO THE SAME SUCH AS IN MELTING AND BOILING POINT.
For e.g - melting of ice is company with absorption of heat whereas conversion of liquid water into ice is accompanied with loss of heat or energy.
THERMOCHEMICAL EQUATION: IT IS A BALANCED STOICHIOMETRIC CHEMICAL EQUATION ALONG WITH PHYSICAL STATES OF REACTANTS AND PRODUCTS AND ENERGY CHANGE REPRESENTED IN THE FORM OF 'DELTA H' WHEN REPRESENT IN A SINGLE EQUATION FORM THE THERMOCHEMICAL EQUATION.
IT IS IMPORTANT TO REPRESENT THE PHYSICAL STATE OF REACTANTS AND PRODUCTS AND PRODUCTS BECAUSE DIFFERENT STATE OF MATTER HAVE DIFFERENT ENERGY
For e.g water is produced in different physical state in same chemical reaction but energy change are different.
EXOTHERMIC REACTION: IT IS A TYPE OF CHEMICAL REACTION IN WHICH LARGE AMOUNT OF ENERGY IS RELEASED.
ENDOTHERMIC REACTION: IT IS A LYPE OF CHEMICAL REACTION IN WHICH LARGE AMOUNT OF ENERGY IS ABSORBED.
THERMOCHEMISTRY: AS CHEMICAL ENERGETICS DEALS WITH THE STUDY OF HEATS CHANGE SIMILARLY THERMOCHEMISTRY DEALS WITH THE STUDY OF ENERGY TRANSFORMATION AS HEAT IS ALSO A FORM OF ENERGY. A REACTION MAY RELEASE OR ABSORB ENERGY , AND A PHASE CHANGE MAY DO THE SAME SUCH AS IN MELTING AND BOILING POINT.
For e.g - melting of ice is company with absorption of heat whereas conversion of liquid water into ice is accompanied with loss of heat or energy.
THERMOCHEMICAL EQUATION: IT IS A BALANCED STOICHIOMETRIC CHEMICAL EQUATION ALONG WITH PHYSICAL STATES OF REACTANTS AND PRODUCTS AND ENERGY CHANGE REPRESENTED IN THE FORM OF 'DELTA H' WHEN REPRESENT IN A SINGLE EQUATION FORM THE THERMOCHEMICAL EQUATION.
IT IS IMPORTANT TO REPRESENT THE PHYSICAL STATE OF REACTANTS AND PRODUCTS AND PRODUCTS BECAUSE DIFFERENT STATE OF MATTER HAVE DIFFERENT ENERGY
For e.g water is produced in different physical state in same chemical reaction but energy change are different.
 |
| THERMODYNAMIC EQUATION |
Comments
Post a Comment