WHAT IS VAPOUR PRESSURE? / FACTORS ON WHICH VAPOUR PRESSURE DEPENDS
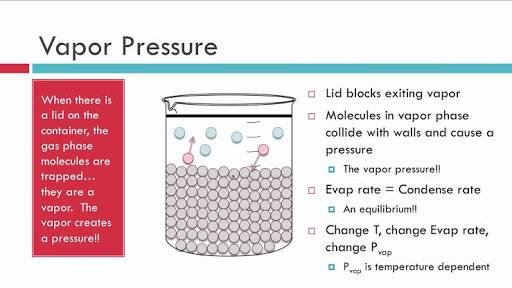
VAPOUR PRESSURE : THE PRESSURE EXERTED BY THE VAPOUR ON THE SURFACE OF THE LIQUID AT EQUILIBRIUM IS CALLED AS VAPOUR PRESSURE.
1. TO EXPLAIN THE CONCEPT OF VAPOUR PRESSURE, LET US TAKE A CLOSED CONTAINER WHICH IS HALF FILLED WITH WATER. THE WATER IN THE CONTAINER IS ALLOWED TO ESCAPE i.e ALLOWED TO EVAPORATE.
2. DUE TO EVAPORIZATION, THE SOME OF THE MOLECULES OF LIQUID WELL GET CONVERTED INTO VAPOUR WITH RESPECT TO TIME , THE AMOUNT OF LIQUID WILL DECREASE AND THE AMOUNT OF VAPOUR INCEREASE.
3. THESE GAS MOLECULES MOVE RANDOMLY IN THE EMPTY SPACE OF THE BEAKER.
4. DUE TO THE RANDOM MOVEMENT, SOME OF THE GAS MOLECULE WILL COME IN CONTACT WITH THE UPPERMOST LAYER OF THE LIQUID AND STARTS CONDENSING.
5. IN THE STARTING , THE RATE OF EVAPORIZATION IS NOT EQUAL TO THE RATE OF CONDENSATION. BUT AFTER SOMETIME , AN EQUILIBRIUM WILL BE FORMED. AND AT EQUILIBRIUM, THE RATE OF EVAPORATION IS EQUAL TO THE RATE OF CONDENSATION.
NOTE : VAPOUR PRESSURE CAN BE CALCULATED ONLY IN CLOSED CONTAINER.
FACTORS ON WHICH VAPOUR PRESSURE DEPENDS :
THERE ARE TWO FACTORS ON WHICH VAPOUR PRESSURE DEPENDS THAT ARE :
1.NATURE OF LIQUID : THE NATURE OF LIQUID IS EXPLAINED ON THE BASIS OF ITS INTERMOLECULAR FORCES. GREATER THE MAGNITUDE OF INTERMOLECULAR FORCES, LESSER WILL BE THE VAPOUR PRESSURE.
2. EFFECT OF TEMPERATURE : VAPOUR PRESSUR IS DIRECTLY PROPORTIONAL TO TEMPERATURE. THIS IS BECAUSE ON INCERASING THE TEMPERATURE THE KINETIC ENERGY OF THE MOLECULE INCREASES. AND DUE TO THE INCREASE IN THE KINETIC ENERGY , THE ESCAPING TENDENCY OF WATER MOLECULE ALSO INCREASE .
1. TO EXPLAIN THE CONCEPT OF VAPOUR PRESSURE, LET US TAKE A CLOSED CONTAINER WHICH IS HALF FILLED WITH WATER. THE WATER IN THE CONTAINER IS ALLOWED TO ESCAPE i.e ALLOWED TO EVAPORATE.
2. DUE TO EVAPORIZATION, THE SOME OF THE MOLECULES OF LIQUID WELL GET CONVERTED INTO VAPOUR WITH RESPECT TO TIME , THE AMOUNT OF LIQUID WILL DECREASE AND THE AMOUNT OF VAPOUR INCEREASE.
3. THESE GAS MOLECULES MOVE RANDOMLY IN THE EMPTY SPACE OF THE BEAKER.
4. DUE TO THE RANDOM MOVEMENT, SOME OF THE GAS MOLECULE WILL COME IN CONTACT WITH THE UPPERMOST LAYER OF THE LIQUID AND STARTS CONDENSING.
5. IN THE STARTING , THE RATE OF EVAPORIZATION IS NOT EQUAL TO THE RATE OF CONDENSATION. BUT AFTER SOMETIME , AN EQUILIBRIUM WILL BE FORMED. AND AT EQUILIBRIUM, THE RATE OF EVAPORATION IS EQUAL TO THE RATE OF CONDENSATION.
NOTE : VAPOUR PRESSURE CAN BE CALCULATED ONLY IN CLOSED CONTAINER.
FACTORS ON WHICH VAPOUR PRESSURE DEPENDS :
THERE ARE TWO FACTORS ON WHICH VAPOUR PRESSURE DEPENDS THAT ARE :
1.NATURE OF LIQUID : THE NATURE OF LIQUID IS EXPLAINED ON THE BASIS OF ITS INTERMOLECULAR FORCES. GREATER THE MAGNITUDE OF INTERMOLECULAR FORCES, LESSER WILL BE THE VAPOUR PRESSURE.
2. EFFECT OF TEMPERATURE : VAPOUR PRESSUR IS DIRECTLY PROPORTIONAL TO TEMPERATURE. THIS IS BECAUSE ON INCERASING THE TEMPERATURE THE KINETIC ENERGY OF THE MOLECULE INCREASES. AND DUE TO THE INCREASE IN THE KINETIC ENERGY , THE ESCAPING TENDENCY OF WATER MOLECULE ALSO INCREASE .

Nycc explaination
ReplyDeleteDoes it depend upon surface area
ReplyDeleteNo this is only a function of temp doesn't de depend upon amount of liquid, surface area or volume
DeleteGood explanation
ReplyDeleteNice 👌 👌👌 👌👌 👌👌 👌 explanation
ReplyDeleteDoes it depends on mole fraction??
ReplyDeleteNice 👍👍👍👍👍👍👍
ReplyDeleteThanks for helping me click here
ReplyDelete